ONTOZRY®▼(cenobamate) Clinical Guidelines

This promotional website is managed and funded by Angelini Pharma and is intended for UK and Ireland healthcare professionals only.
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events and product complaints should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK or www.hpra.ie for Ireland. Adverse events and product complaints should also be reported to Angelini Pharma on (UK) +44 2034889643, (IRE) +353 1 584 4671 or UKIReporting@angelinipharma.com

The use of ONTOZRY® (cenobamate) is recommended by NICE Technology Appraisal TA753
ONTOZRY® (cenobamate) is approved for use in focal epilepsy by NHS Scotland
NICE Guideline (NG217) on seizure management
Educational support for informed prescribing
ONTOZRY® is an adjunctive therapy for adults with refractory focal-onset seizures who remain uncontrolled despite two or more anti-seizure medications (ASMs). NICE recommends ONTOZRY®as a treatment option for this patient group in England and Wales.1 The drug is also approved for use in Scotland; however, restrictions apply to its use here.2
The use of ONTOZRY® (cenobamate) is recommended by NICE Technology Appraisal TA753
NICE endorses ONTOZRY® as a treatment option for focal-onset seizures in adults with or without secondary generalisation.1
NICE guidance states ONTOZRY® is recommended if:
- after at least 1 first-line add-on treatment has not controlled seizures, and other first-line add-on treatments are contraindicated or not tolerated, and
- treatment is started by a healthcare professional with expertise in epilepsy, after which treatment can be continued in primary care.
ONTOZRY® has demonstrated in clinical trials that with standard of care (SoC), it has the potential to significantly reduce seizure frequency in most patients, as well as offering the potential of achieving seizure freedom (100% reduction) in some patients whilst being generally well tolerated. The C017 and C021 clinical studies showed that ONTOZRY® has a safety profile characterised by tolerable side effects , with the majority of adverse events ranging from mild to moderate in severity, and are dose-dependent.7,8
ONTOZRY® has a dual complementary mechanism of action, with potential to both prevent seizure initiation and limit seizure spread.1 The precise mechanism of action by which cenobamate exercises its therapeutic effects in patients with focal-onset seizures is unknown.
ONTOZRY® (cenobamate) is approved for use in focal epilepsy by NHS Scotland
NHS Scotland approved the restricted use of ONTOZRY® as an adjunctive therapy. ONTOZRY® is also endorsed by the Scottish Medicines Consortium (SMC) for the use in adult patients with focal-onset seizures, with or without secondary generalisation, who have not been adequately controlled despite treatment with at least two ASMs.2
Approvals were based on clinical evidence that demonstrated ONTOZRY® was superior to placebo (+SoC) in reducing the frequency of focal-onset seizures by >50%, in patients with uncontrolled focal seizures despite treatment with ASMs.1
NICE Guideline (NG217) on seizure management
The April 2022 NICE guideline (NG217) focuses on enhancing the diagnosis and treatment of various seizure types and epilepsy syndromes in children, young adults, and adults. It's designed to mitigate risks associated with epilepsy and is specifically tailored for healthcare professionals and people living with epilepsy.9
NICE guidance for epilepsy self-management
The NICE guidelines encourage healthcare providers (HCPs) to support individuals with epilepsy and their families and caregivers to confidently self-manage their condition and make informed treatment decisions.9
To aid the patient in understanding their epilepsy, key topics that could be discussed include specific epilepsy syndrome or seizure types, common seizure triggers, the impact on daily activities, and strategies to reduce epilepsy-related risks, including Sudden Unexpected Death in Epilepsy (SUDEP).9
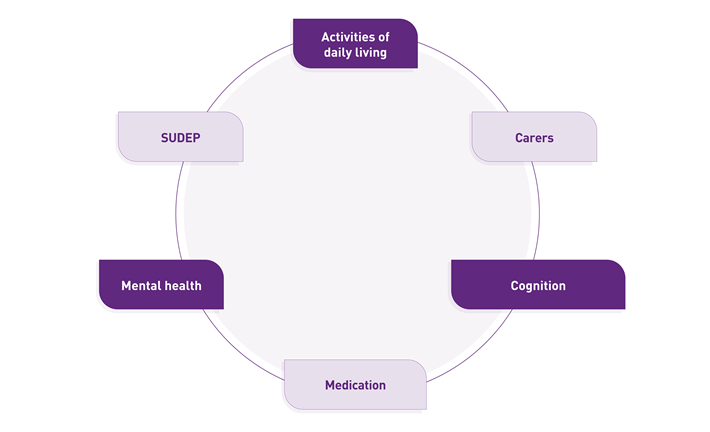
To aid the patients in effectively managing their condition, NICE guidance for epilepsy recommends that HCPs share advice on the following topics:
Daily Living and safety considerations
Epilepsy affects everyday life, and special safety considerations should be taken to prevent accidents and injuries.9
Safety precautions: patients are advised to prioritise activities such as showering instead of bathing, being cautious in the kitchen, ensuring the safety of infants and children, and avoiding work at height.9
Child and youth safety: children and young people require supervision during activities such as swimming, water sports, and climbing to ensure their safety.9
Driving regulations: HCPs are encouraged to discuss the implications of epilepsy on the eligibility to drive.9 Individuals with epilepsy are generally allowed to drive with a group 1 driving licence if they have been seizure-free for at least 12 months. If seizures occur, the individual must stop driving and inform DVLA (UK)10 or consult a medical professional (Ireland).11
Work and education: HCPs should address common challenges and legal rights in the realm of employment and education.9
Carers
Supporting someone with epilepsy often poses a challenge for caregivers and takes a physical and emotional toll. Support for carers needs to be accessible, and the resources tailored to address the specific needs of the caregivers.9
Cognition
Epilepsy and ASMs not only impact the body but can also impact cognitive function. Patients may experience deficits in memory, attention, and concentration, which can reduce productivity and focus. Special adjustments at school or the workplace might be necessary, for instance, additional time for assignments.9
Medication
Adherence to ASMs is critical to reduce the risk of seizures and SUDEP. Strategies to support the individual can include using pill organisers and establishing a routine.12 ASMs can lead to medication-related side effects, namely mood changes, dizziness, and fatigue.13 Side effects of ASMs should be discussed as soon as they appear and, if necessary, the treatment adjusted.
To promote adherence to the medication, HCPs should always provide clear explanations when changes to a medication are made.9
Mental health
An epilepsy diagnosis can be accompanied by depression, anxiety, or low mood. HCPs should recognise the emotional and psychological impact epilepsy can have. Epilepsy-related stigma can also impact their mental health. Furthermore, epilepsy is commonly associated with neurobehavioural conditions, namely autism and ADHD.9
SUDEP
Individuals with epilepsy, their families, and carers frequently express concerns about SUDEP. HCPs should discuss these concerns with patients and carers and provide information on SUDEP. In particular, an emphasis should be placed on explaining the risk factors and ways to mitigate them as well as providing access to relevant SUDEP counselling and support services.9
Referral to tertiary epilepsy services
According to the NICE guidance NG217 for epilepsy, patients should be referred to a tertiary epilepsy service within 4 weeks if:
- the diagnosis is uncertain or atypical
- seizures are not adequately controlled despite appropriate treatment
- further assessment or specialised interventions are required, e.g., surgery, vagus nerve stimulation (VNS), ketogenic diet, EEG telemetry) becomes apparent
- individuals meet eligibility criteria and express interest in participating in a clinical trial or research study.9

Non-pharmacological treatments1
NICE guidance NG217 for epilepsy includes recommendations on non-pharmacological treatments:
-
- A ketogenic diet may be considered under the supervision of a tertiary epilepsy specialist for individuals with specific childhood-onset epilepsy syndromes or drug-resistant epilepsy, where other treatments have failed or are unsuitable
- Resective epilepsy surgery for appropriate patients
- Vagus nerve stimulation (VNS) for individuals with drug-resistant epilepsy.9
Please see the full NICE guidelines for details of non-pharmacological treatments.1
Individualised treatment strategy
According to NG217, the treatment plan should be tailored to each patient or their caregivers based on:
- Age, sex, and personal circumstances
- Individual preferences
- Seizure type and specific epilepsy symptoms
- Whether treatment is necessary. If treatment is indicated, the optimal treatment regimen for each patient needs to be determined
- The risks and benefits of ASMs, including their importance in reducing the risk of epilepsy-related death.
- Comorbidities and potential interactions with other medications.9
Learn about dosing and titration of ONTOZRY®▼ (cenobamate) to better support your patients' treatment decisions.

Minimising risk of epilepsy-related death (including SUDEP)
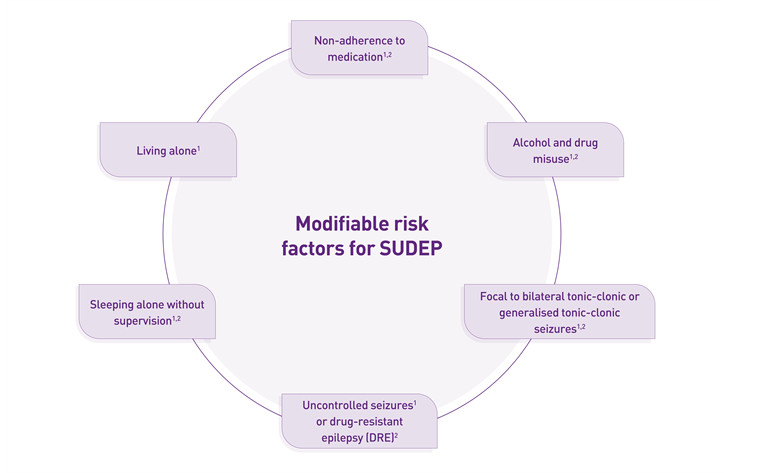
NICE NG217 advises discussing SUDEP from the time of diagnosis and continuing throughout care. To reduce the risk of SUDEP and provide optimal patient care, HCPs need to recognise risk factors such as uncontrolled seizures or drug-resistant epilepsy, focal to bilateral tonic-clonic or generalised tonic-clonic seizures. In addition, further modifiable risk factors include living alone and sleeping without supervision. It is also important to communicate the risks of non-adherence to ASMs and alcohol or recreational drug misuse with the patients.9

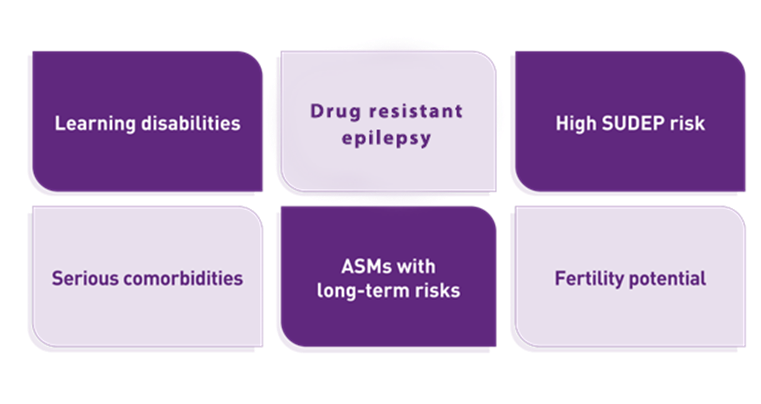
Monitoring and review
Certain patient groups need to be reviewed annually. This group consists of patients with learning disabilities, drug-resistant epilepsy, significant comorbidities, and increased risk of SUDEP. Individuals who take ASMs with potential long-term safety profile concerns and fertility or reproductive health considerations should also be assessed every year.9
Educational support for informed prescribing
Visit our resource page for ONTOZRY® and explore downloadable materials, clinical summaries, and educational tools to support you in evidence-based decision-making in practice.
Abbreviations
ADHD, attention deficit hyperactivity disorder; ASM, anti-seizure medication; BNF, British National Formulary; DVLA, Driver and Vehicle Licensing Agency; EEG, electroencephalogram; HCP, healthcare professional; NICE, National Institute for Health and Care Excellence; SMC, Scottish Medicines Consortium; SUDEP, sudden unexpected death in epilepsy; VNS, vagus nerve stimulation (VNS); SoC, Standard of Care.

Footnotes
© NICE 2021 Cenobamate for treating focal onset seizures in epilepsy. Technology appraisal guidance TA753. Available from https://www.nice.org.uk/guidance/ta753. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.1
© SMC 2022 Advice available from https://www.scottishmedicines.org.uk/medicines-advice/cenobamate-ontozry-full-smc2408. All rights reserved. Subject to Notice of rights. SMC advice is prepared for the National Health Service in Scotland. SMC accepts no responsibility for the use of its content in this product/publication.2
- NICE. Cenobamate for treating focal onset seizures in epilepsy. Available at: https://www.nice.org.uk/guidance/ta753 (last accessed November 2025)
- SMC. Cenobamate (Ontozry). Available at: https://scottishmedicines.org.uk/medicines-advice/cenobamate-ontozry-full-smc2408/ (last accessed November 2025)
- BNF. Cenobamate. Available at: https://bnf.nice.org.uk/drugs/cenobamate/ (last accessed November 2025)
- Hertfordshire and West Essex ICB.Cenobamate. Available at: https://www.hweclinicalguidance.nhs.uk/prescribing-guidance/cenobamate/ (last accessed November 2025)
- Derbyshire ICBs. Available at: https://www.derbyshiremedicinesmanagement.nhs.uk/medicines-management/full_traffic_light_classification/show_drug/cenobamate/ (last accessed November 2025)
- European Medicines Agency (EMA). Ontozry. Available at: https://www.ema.europa.eu/en/medicines/human/EPAR/ontozry. (last accessed November 2025)
- Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38-48. doi:10.1016/S1474-4422(19)30399-0
- Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099-1108. doi:10.1111/epi.16525
- NICE. Epilepsies in children, young people and adults. Available at: https://www.nice.org.uk/guidance/ng217/resources/epilepsies-in-children-young-people-and-adults-pdf-66143780239813 (last accessed November 2025)
- DVLA. Epilepsy Fact sheet. Available at: https://assets.publishing.service.gov.uk/media/5a7eef1440f0b6230268c73e/INS9.pdf (last accessed November 2025)
- NDLS. Epilepsy, Seizures and Driving. Available at: https://www.ndls.ie/images/Documents/Guidelines/10421_Epilepsy_Seizures_and_Driving_DL_(hi-res_screen).pdf (last accessed November 2025)
- NICE. Medicines adherence: involving patients in decisions about prescribed medicines and supporting adherence. Available at: https://www.nice.org.uk/guidance/cg76/chapter/Recommendations (last accessed November 2025)
- Barnard SN, Chen Z, Kanner AM, et al. The adverse effects of commonly prescribed antiseizure medications in adults with newly diagnosed focal epilepsy. Neurology. 2024;103(7):e209821. doi:10.1212/WNL.0000000000209821

MAT-UKI-0268-P | November 2025
 HarmoniaMentis
HarmoniaMentis
