Long term efficacy and retention with ONTOZRY®▼ (cenobamate)

▼ This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events and product complaint should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK or www.hpra.ie for Ireland. Adverse events and product complaint should also be reported to Angelini Pharma on (UK) +44 2034889643, (IRE) +353 1 584 4671 or UKIReporting@angelinipharma.com
This page is intended for United Kingdom and Ireland healthcare professionals.

Retention with adjunctive ONTOZRY®
The NICE guideline for diagnosing and managing epilepsy highlights the importance of supporting people with epilepsy to take their medications as prescribed to reduce seizures.1
Non-adherence to medication increases the risk of seizures and is a potentially modifiable risk factor for sudden unexpected death in epilepsy (SUDEP).1
Poor adherence to anti-epileptic drugs is associated with:2
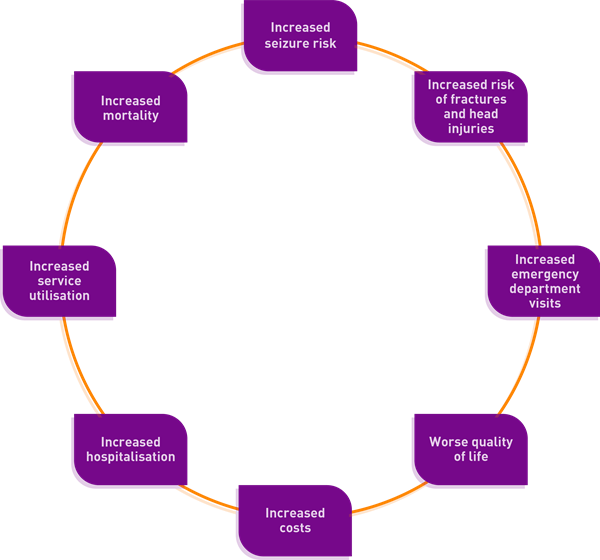
Reasons for poor adherence are varied but may include:2,3
- Beliefs about medications
- Feeling depressed or anxious
- Poor self-management
- Side effects
- Forgetfulness
- Poor physician–patient relationship
- Poor social support
It is not easy to accurately measure adherence4 but the rate of non-adherence in adults with epilepsy is estimated to be between 29-66%.3*
Retention rates for anti-seizure medications (ASMs) provide a composite measure of efficacy, tolerability, safety and adherence over a defined time period,5 and their use as an outcome measure is recommended by the European Medicines Agency (EMA) as a global indicator of a drug's clinical effectiveness.5
Data from retention studies may also provide benchmarks for interpreting and comparing clinical efficacy of ASMs, as well as evaluating long-term efficacy and tolerability beyond controlled clinical trials.5

High rates of retention on ONTOZRY® were observed during the clinical development programme5
Retention rates on ONTOZRY® appear to be comparable to or better than rates reported for ASMs in pre-marketing open-label extension studies conducted as part of respective clinical development programmes.6,7,8

- High long-term retention rates with ONTOZRY® suggests sustained clinical efficacy and tolerability.5
- Concomitant anti-seizure medications did not impact retention.5
With ONTOZRY® long-term retention rates were stable across Years 3–69
- Retention rates with ONTOZRY® were higher than those reported for other ASMs in open-label extension (OLE) studies in patients with focal seizures.9,†
- Around 61% of patients continue with ONTOZRY® for at least 4 years.
These retention rates support the acceptability of the titration schedule and compliance with ONTOZRY®10

Glossary:
Compliance: The extent to which the patient’s behaviour matches the prescriber’s recommendations.11
Adherence: The extent to which the patient’s behaviour matches agreed recommendations from the prescriber.11
Retention: The extent to which the patient is retained on the medication.11
Abbreviations:
AED = Anti-epileptic drug; ASM = anti-seizure medication; BRV = brivaracetam; CNB = cenobamate; EMA = European Medicines Agency; LAC = lacosamide; LEV = levetiracetam; NICE = National Institute for Health and Care Excellence; OLE = open-label extension; PER = perampanel; SUDEP = sudden unexpected death in epilepsy.
Footnotes:
*Data from a systematic review of adherence studies in adults with epilepsy who were prescribed AEDs.3
†Comparing participant retention rates between ASM clinical trials can be challenging due to the lack of head-to-head comparison studies and the broad time range over which they have been performed. Studies may also have differences in populations, follow-up and concomitant ASMs.
© NICE 2022 Epilepsies in children, young people and adults. Available from https://www.nice.org.uk/guidance/ng217. All rights reserved. Subject to Notice of rights. NICE guidance is prepared for the National Health Service in England. All NICE guidance is subject to regular review and may be updated or withdrawn. NICE accepts no responsibility for the use of its content in this product/publication.
- NICE. Epilepsies in children, young people and adults. NICE guideline 217. 27 April 2022. Available at: https://www.nice.org.uk/guidance/ng217 (last accessed November 2024).
- Al-Aqeel S, et al. Cochrane Database Syst Rev. 2017;2(2):CD008312.
- O’Rourke G, O’Brien JJ. Seizure. 2017;45:160-8.
- Javor A, et al. R Soc Open Sci. 2019;6:180850.
- Sander JW, et al. Epilepsia. 2022;63(1):139-49.
- Chung SS. et al. Neurology. 2020;94(22):2311-22.
- Krauss GL, et al. Lancet Neurol. 2020;19(1):38-48 and supplementary appendix.
- Sperling MR, et al. Epilepsia. 2020;61(6):1099-108.
- French JA, et al. Epilepsia. 2021;62(9):2142-50.
- Steinhoff BJ, et al. Epilepsy Behav. 2021;123:108270.
- Report for the National Co-ordinating Centre for NHS Service Delivery and Organisation R & D (NCCSDO). Concordance, adherence and compliance in medicine taking. Available from: https://njl-admin.nihr.ac.uk/document/download/2027234 (last accessed November 2024).

MAT-UKI-0057-P | November 2024
 HarmoniaMentis
HarmoniaMentis