ONTOZRY®▼(cenobamate) Tolerability & Side Effects

This promotional website is managed and funded by Angelini Pharma and is intended for UK and Ireland healthcare professionals only.
▼This medicinal product is subject to additional monitoring. This will allow quick identification of new safety information. Adverse events and product complaint should be reported. Reporting forms and information can be found at www.mhra.gov.uk/yellowcard for the UK or www.hpra.ie for Ireland. Adverse events and product complaint should also be reported to Angelini Pharma on (UK) +44 2034889643, (IRE) +353 1 584 4671 or UKIReporting@angelinipharma.com

ONTOZRY® (cenobamate) side effects
ONTOZRY® (cenobamate) contraindications and special warnings
Use of ONTOZRY® (cenobamate) in special populations
The pivotal study (C017): tolerability and side effects of ONTOZRY® (cenobamate)
The supportive study (C013): ONTOZRY® (cenobamate) side effects
The C021 study and the tolerability and side effects of ONTOZRY® (cenobamate)
Tolerability resources for clinical support
ONTOZRY® is an anti-seizure medication (ASM) indicated for the adjunctive treatment of focal-onset seizures with or without secondary generalisation in adult patients with epilepsy who have not been adequately controlled despite treatment with at least 2 anti-epileptic medicinal products. ONTOZRY® has a dose-dependent adverse event (AE) profile.1,2
The prescribing doctor must understand the drug’s AE profile, contraindications, and key safety warnings to formulate an informed treatment plan.
ONTOZRY® (cenobamate) side effects
ONTOZRY® is generally well-tolerated by patients. The majority of treatment-emergent adverse events (TEAEs) for ONTOZRY® are dose-dependent and characterised by a mild or moderate severity.1–3
The most commonly observed side effects of cenobamate are somnolence (including sedation and hypersomnia), dizziness, fatigue, and headache. Continued monitoring and patient counselling are critical safeguards to ensure successful tolerability outcomes.1–3
Tabulated list of ONTOZRY® (cenobamate) adverse effects

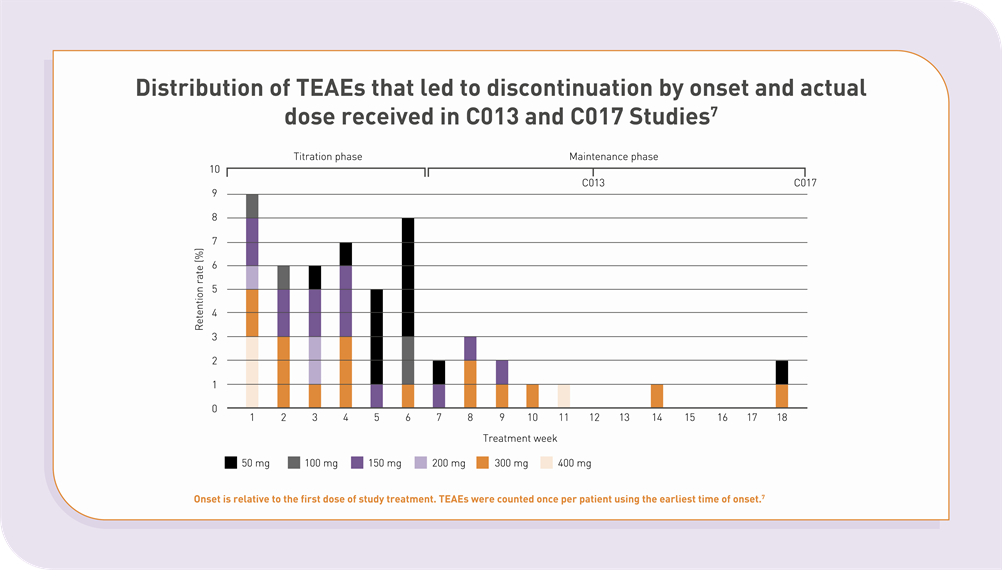
Most of ONTOZRY® (cenobamate) side effects occurred during titration
Clinical studies support the use of ONTOZRY® as an adjunctive treatment option for adult patients with drug-resistant focal epilepsy.4 The pivotal C017 study revealed that the majority of side effects of cenobamate lead to discontinuation during the titration phase.4
C017 showed that adverse events were more common in patients who were treated with the higher doses.4 A gradual dosing and titration schedule has been reported to reduce both the frequency and severity of TEAEs compared to faster titration approaches.5,6
Study C021, where the “start low, go slow” titration strategy was implemented, proved to be an effective approach to enhance tolerability.5,6
Distribution of TEAEs that led to discontinuation by onset and actual dose received in C013 and C017 studies

ONTOZRY® (cenobamate) contraindications and special warnings
Cenobamate can cause rare but serious side effects, including suicidal ideation, drug reaction with eosinophilia and systemic symptoms (DRESS), and QT-shortening. A gradual titration of the drug and vigilance are critical mitigating measures to reduce the risk. It is also important to acknowledge that ONTOZRY® is contraindicated in patients with hypersensitivity to the active substance or to any of the excipients or those with Familial Short-QT syndrome.1,2
-
Suicidal thoughts and behaviours have been observed in patients receiving ASMs, including ONTOZRY®. However, the underlying mechanism driving this risk remains unclear.1,2
The precise mechanism of action of ONTOZRY® is not yet fully established, and current data also do not rule out a potential risk for suicidal ideation associated with the drug. It is therefore essential to closely monitor patients for signs of suicidal thoughts and behaviours. Healthcare providers (HCPs) should emphasise to patients and caregivers to seek medical attention if such symptoms appear.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
The association between rapid titration and a higher risk of DRESS is well documented. There is a higher risk of DRESS when treatment is initiated at a high dose and titrated weekly or more frequently. Importantly, C021 showed that in 1,339 patients, no DRESS cases occurred with a slow 12.5 mg/day and 2-week interval titration.1,2,4
HCPs and patients should monitor for early signs of DRESS, such as fever, rash, lymphadenopathy, and hepatic abnormalities. Early manifestations of hypersensitivity may be present even though a rash is not evident. DRESS can be life-threatening or fatal. ONTOZRY® must therefore be discontinued immediately if DRESS is suspected, and alternative treatment should be explored.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
While ONTOZRY® can cause a dose-dependent shortening of the QTcF interval, no reductions in the QTcF interval below 340 msec were observed in clinical trials. Furthermore, clinical trial data did not show evidence of additional QT shortening when ONTOZRY® was used alongside other anti-epileptic drugs.1,2
Caution is always advised when prescribing cenobamate with other medications known to shorten the QT interval, such as certain antipsychotics or antiarrhythmics. ONTOZRY® is contraindicated in patients with Familial Short-QT syndrome.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
ONTOZRY® contains lactose and may not be suitable for patients with rare hereditary disorders such as galactose intolerance, total lactase deficiency, or glucose-galactose malabsorption. To prevent potential AEs, HCPs should assess for these conditions before initiating therapy.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
ONTOZRY® is contraindicated in patients with hypersensitivity to cenobamate or any of the excipients, including:1,2
- lactose monohydrate
- magnesium stearate (E470b)
- microcrystalline cellulose (E460)
- silica, colloidal anhydrous (E551)
- sodium starch glycolate
- indigo carmine aluminium lake (E132)
- iron oxide red (E172)
- iron oxide yellow (E172)
- macrogol
- partially hydrolysed poly (vinyl alcohol) (E1203)
- talc (E553b)
- titanium dioxide (E171).1,2
See the Summary of Product Characteristics for full prescribing information.1,2

Use of ONTOZRY® (cenobamate) in special populations
ONTOZRY® is not indicated for the adjunctive treatment of focal-onset seizures in paediatric patients. The drug requires caution in certain adult groups, and specific recommendations for special population groups with certain conditions or circumstances apply:1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
Dose adjustments should be considered in patients with renal impairment, as the maximum recommended dose in these patients is 300 mg/day. ONTOZRY® is contraindicated for patients with end-stage renal disease or those undergoing haemodialysis.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
Chronic hepatic disease causes an overexposure to ONTOZRY®. While no adjustment to the initial dose is necessary, a reduction of the target dose by up to 50% may be considered. The maximum recommended dose for patients with mild or moderate hepatic impairment is 200 mg/day. ONTOZRY® is not recommended for use in patients with severe hepatic impairment.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
There is no need for dose adjustment of cenobamate based on weight gain unless the patient’s weight increases by more than 30% of their initial body weight, or if there is comorbid renal or hepatic impairment.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
The number of patients aged 65 years and older in clinical studies of ONTOZRY® was insufficient to determine whether their response differed from that of younger adults. Elderly patients taking anti-epileptic medications are known to experience a higher incidence of AEs, such as fatigue, gait disturbance, falls, ataxia, balance disorder, dizziness, and somnolence.1,2
Therefore, dose selection in older adults should be approached with caution, and the initial treatment should typically be started at the lower end of the dosing range. This approach takes into account the increased likelihood of reduced hepatic or renal function, coexisting medical conditions, and potential drug interactions of cenobamate in polymedicated individuals.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
Fertility
The impact of cenobamate on human fertility is unknown, as animal studies are inconclusive due to cenobamate exposure levels below those seen in clinical use. ONTOZRY® is not recommended for women of childbearing potential who are not using contraception. Women of reproductive age taking oral contraceptives should use additional or alternative non-hormonal methods of contraception during treatment and for 4 weeks after stopping ONTOZRY®.1,2
Pregnancy
The risk of congenital malformations in children born to women with epilepsy is two to three times higher than the baseline rate of ~3% in the general population.1,2 The increased malformation rates appear to be higher in women receiving anti-epileptic polytherapy, although it is unclear whether this is due to the medication, the underlying condition, or both.1,2
Discontinuation of treatment during pregnancy may worsen the disease, which can pose risks to both the mother and the foetus.7
There are no adequate data on the use of ONTOZRY® during pregnancy in humans, but animal studies have shown that cenobamate crosses the placenta and causes reproductive toxicity at exposures below the clinical level.1,2
ONTOZRY® should only be used during pregnancy if the potential benefit to the mother outweighs the potential risk to the foetus.1,2
Lactation
Animal studies have shown that cenobamate is excreted in the milk of lactating rats. Yet, it is unknown whether cenobamate or its metabolites are excreted in human breast milk. Due to the potential risk, breastfeeding should be discontinued during treatment with ONTOZRY®, as AEs cannot be ruled out.1,2
See the Summary of Product Characteristics for full prescribing information.1,2
-
The safety profile and effectiveness of ONTOZRY® in patients less than 18 years of age has not been established. No data are available.1,2
See the Summary of Product Characteristics for full prescribing information.1,2

The pivotal study (C017): tolerability and side effects of ONTOZRY® (cenobamate)
The efficacy of ONTOZRY® was evaluated in several clinical trials . C017 was a multicentre, randomised, double-blind, placebo-controlled dose-response study. The study found that most TEAEs were mild or moderate in severity.3,8
The majority of patients experienced at least one TEAE irrespective of the assigned treatment:3
- 70% (76 of 108) in the placebo
- 65% (70 of 108) in the 100 mg ONTOZRY® group
- 76% (84 of 110) in the 200 mg ONTOZRY® group
- 90% (100 of 111) in the 400 mg ONTOZRY® group.
The most frequent TEAEs - including somnolence, dizziness, headache, diplopia, and fatigue - were experienced by more than 10% of patients.3
No deaths were associated with the treatment, but a total of 37 serious TEAEs occurred in 28 patients. The serious TEAEs that occurred in more than one patient in any treatment group were: seizures (n=2), ataxia (n=2), dizziness (n=2), nystagmus (n=2), and suicidal ideation. In addition, 1 suicide attempt was described.
53 patients discontinued treatment due TEAEs.3
Hypersensitivity reactions were characterised by a rash accompanied by involvement of one or more other body systems. Hypersensitivity reactions were reported in 3 patients, all of whom recovered after discontinuing treatment. The reported events included DRESS (n=1), pruritic rash with pyrexia (n=1), and rash with facial swelling (n=1).3
C017 Open-label extension (OLE) reported side effects
Patients who completed the 18-week double-blind study (n=360) could enter the OLE. The safety population comprised 355 patients, including 265 originally randomised to ONTOZRY® and 90 originally randomised to placebo. The OLE safety data showed that 88.2% (313/355) of patients experienced TEAEs during the OLE.
TEAEs reported in ≥10% of patients in any group were dizziness, somnolence, fatigue, headache, diplopia, gait disturbances, and upper respiratory tract infection.9
Commonly described TEAEs appeared mostly within the first month of the treatment during the OLE conversion. Serious TEAEs occurred in 20.3% (n=72/355) of patients. Seizure (n=5) and vertigo (n=4) were the only serious TEAEs in >1% of patients. No deaths were attributed to the drug.9
TEAEs that led to discontinuation occurred in 8.7% (n=31/355) of patients, including:9
- Nervous system disorders (n=12): dizziness (n=3), somnolence (n=2), balance disorder (n=2), and depression (n=2).
- Psychiatric disorders (n=6): depression (n=2), bradyphrenia, hallucination, persistent depressive disorder, and psychotic disorder (all n=1).
- Skin and subcutaneous tissue disorders (n=6): alopecia, angioedema, pruritus, maculopapular rash, skin lesion, and toxic skin eruption (all n=1).
- Others occurring in >1 patient: infections and infestations (n=2), and eye disorders (n=2).
At the data cut-off, six deaths had been recorded during the OLE due to pneumonia/sepsis, septicaemia, fatal injuries after being struck by a car, cardiogenic shock, myocardial infarction, and suicide. The investigator assessed these deaths as unrelated to ONTOZRY®.9
The supportive study (C013): ONTOZRY® (cenobamate) side effects
C013 was a multicentre, randomised, double-blind, placebo-controlled efficacy and safety profile study. The study showed that the identified TEAEs were mild or moderate in severity.10 The most frequently (>10 % of patients) TEAEs included:10
- Somnolence (22.1%)
- Dizziness (22.1%)
- Headache (12.4%)
- Nausea (11.5%)
- Fatigue (10.6%).
Five patients discontinued treatment due to side effects of cenobamate, such as tachycardia, gastroesophageal reflux disease, drug hypersensitivity, nystagmus, aggression, depression, and dyspnoea, with some patients experiencing more than one event.10
Two patients receiving ONTOZRY® experienced serious TEAEs including drug hypersensitivity reaction (n=1) and urinary tract infection (n=1).10
No other serious dermatological TEAEs such as DRESS or Stevens-Johnson syndrome were observed. Additionally, no deaths occurred during the double-blind treatment period.10
C013 OLE reported side effects
All patients who completed the 12-week double-blind study were eligible for the OLE (n=149). At the time of data cut-off, 57% of patients (85 out of 149) still participated in the OLE, with a median treatment duration of 6.8 years (range: 6.4–7.8 years).11
The reported TEAEs were mostly mild or moderate and affected 89.3% of patients (133/149).11 The safety data during the 7.8-year duration of the OLE, included:11
- The most frequently described TEAEs were dizziness (32.9%, 49/149), headache (26.8%, 40/149), and somnolence (21.5%, 32/149).
- Serious TEAEs were reported for 25.5% of patients (38/149), with the most frequent events (>1% of patients) being seizures (n=6), and vomiting, pneumonia, sepsis, and osteoarthritis (each n=2).
Three deaths were recorded during the OLE: SUDEP, cardiac arrest, and suicide (each n=1).
- A treatment stop due to TEAEs occurred in 10.1% of patients (15/149), with the most common symptoms (>1% of patients) being fatigue (1.3%, n=2), ataxia (1.3%, n=2), and memory impairment or amnesia (1.3%, n=2).
The C021 study and the tolerability and side effects of ONTOZRY® (cenobamate)
The CO21 clinical trial was an open-label Phase III safety study involving 1,339 adults with uncontrolled focal seizures who were receiving stable doses of 1–3 ASMs.5
ONTOZRY® treatment began at 12.5 mg/day and was titrated every two weeks to 25, 50, 100, 150, and 200 mg/day, with optional biweekly increases of 50 mg/day permitted up to a maximum of 400 mg/day.5 Most TEAEs were mild or moderate, accounting for 77.8% (1,042/1,339) of all TEAEs.5 Early safety data findings in the interim analysis indicate:5
- 84.2% of patients (1,128/1,339) experienced at least one TEAE, with the most commonly reported events being somnolence (28.1%), dizziness (23.6%), and fatigue (16.6%).
- 189 patients (14.1%) developed skin and subcutaneous tissue disorders.
- 108 patients (8.1%) experienced serious TEAEs with the most frequent being seizures (n=14), epilepsy (n=5), and pneumonia, falls, and dizziness (each n=4).
- More than two patients presented with vomiting, appendicitis, mental status change, suicide attempt, and papular rash (each n=3).
- Six patients developed serious skin and subcutaneous tissue disorders, including allergic dermatitis, erythema, rash, maculopapular rash, facial swelling, and urticaria (at least n=1 each).
More than 1% of patients developed psychiatric side effects due to cenobamate, and these included anxiety (n=31), irritability (n=29), insomnia (n=27), depression (n=26), and confusional state (n=16).5
At data cut-off, four deaths were recorded, comprising sudden death, intracerebral haemorrhage following a fall, fatal injuries after being struck by a car, and respiratory failure in a patient with Angelman syndrome.5
TEAEs leading to treatment discontinuation occurred in 11% of patients (n=147). The most frequently reported TEAEs that caused a treatment stop were:5
- Nervous system disorders (n=45): dizziness (n=14), seizure (n=9), and somnolence (n=9)
- Skin and subcutaneous tissue disorders (n=44): rash (n=9), and rash erythematous, papular rash, pruritus, and urticaria (each n=3).
In addition, one patient discontinued due to a hypersensitivity reaction, and no cases of DRESS were identified.5
Tolerability resources for clinical support
Stay up to date with the prescribing information and safety profile updates for ONTOZRY® to make well-informed treatment decisions. Our clinical guidelines and supporting resources provide comprehensive insights into its clinical use.
Abbreviations:
ASM, anti-seizure medication; AE, adverse events; DRESS, drug reaction with eosinophilia and systemic symptoms; HCP, healthcare providers; TEAEs, treatment-emergent adverse events; OLE, Open-label extension
References
- EMA. ONTOZRY® EU Summary of Product Characteristics. Available at: https://www.ema.europa.eu/en/documents/product-information/ontozry-epar-product-information_en.pdf (last accessed July 2025)
- EMC. ONTOZRY® Summary of Product Characteristics. Available at: https://www.medicines.org.uk/emc/product/13012/smpc (last accessed July 2025)
- Krauss GL, Klein P, Brandt C, et al. Safety and efficacy of adjunctive cenobamate (YKP3089) in patients with uncontrolled focal seizures: a multicentre, double-blind, randomised, placebo-controlled, dose-response trial. Lancet Neurol. 2020;19(1):38-48. doi:10.1016/S1474-4422(19)30399-0
- Steinhoff BJ, Rosenfeld WE, Serratosa JM, et al. Practical guidance for the management of adults receiving adjunctive cenobamate for the treatment of focal epilepsy-expert opinion. Epilepsy Behav. 2021;123(108270):108270. doi:10.1016/j.yebeh.2021.108270
- Sperling MR, Klein P, Aboumatar S, et al. Cenobamate (YKP3089) as adjunctive treatment for uncontrolled focal seizures in a large, phase 3, multicenter, open-label safety study. Epilepsia. 2020;61(6):1099-1108. doi:10.1111/epi.16525
- Steinhoff BJ, Ben-Menachem E, Brandt C, et al. Onset of efficacy and adverse events during Cenobamate titration period. Acta Neurol Scand. 2022;146(3):265-275. doi:10.1111/ane.13659
- Patel SI, Pennell PB. Management of epilepsy during pregnancy: an update. Ther Adv Neurol Disord. 2016;9(2):118-129. doi:10.1177/1756285615623934
- Krauss GL, Chung SS, Ferrari L, Stern S, Rosenfeld WE. Cognitive and psychiatric adverse events during adjunctive cenobamate treatment in phase 2 and phase 3 clinical studies. Epilepsy Behav. 2024;151(109605):109605. doi:10.1016/j.yebeh.2023.109605
- Klein P, Aboumatar S, Brandt C, et al. Long-term efficacy and safety from an open-label extension of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2022;99(10):e989-e998. doi:10.1212/wnl.0000000000200792
- Chung SS, French JA, Kowalski J, et al. Randomized phase 2 study of adjunctive cenobamate in patients with uncontrolled focal seizures. Neurology. 2020;94(22):e2311-e2322. doi:10.1212/WNL.0000000000009530
- French JA, Chung SS, Krauss GL, et al. Long-term safety of adjunctive cenobamate in patients with uncontrolled focal seizures: Open-label extension of a randomized clinical study. Epilepsia. 2021;62(9):2142-2150. doi:10.1111/epi.17007

MAT-UKI-0270-P | October 2025
 HarmoniaMentis
HarmoniaMentis